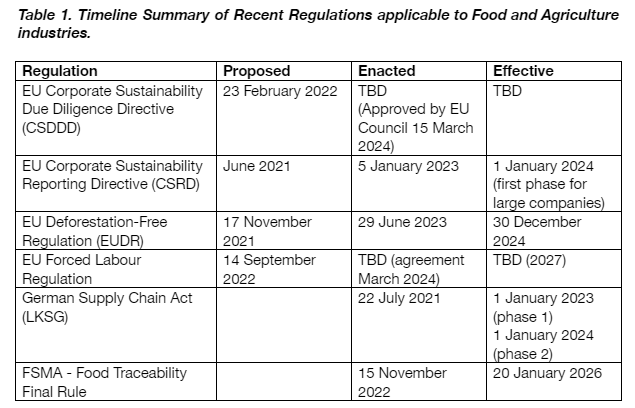
In today’s food supply chain, ensuring transparency, accountability, and sustainability throughout supply chains is paramount, particularly in the food and agriculture industries. Governments and regulatory bodies worldwide have recognized the necessity for stringent measures to tackle issues ranging from forced labour and deforestation to food safety and corporate sustainability. This article explores six pivotal regulations concerning traceability that impact businesses in both the European Union (EU) and the United States (US), shedding light on their objectives, applicability, and the pressing need for adopting innovative technologies.
EU Corporate Sustainability Due Diligence Directive (CSDDD/CS3D)
Objective: The CS3D aims to promote responsible and sustainable corporate behaviour by integrating human rights and environmental considerations into business operations and governance. Its objective is to identify, mitigate, prevent, and account for adverse impacts such as child labour, labour exploitation, environmental contamination, and biodiversity loss across operations and supply chains.
Key Measures: Under the CS3D, businesses will be required to conduct due diligence, risk assessments, and take appropriate measures to address identified risks. This includes implementing transparent policies, adopting auditable traceability systems, and ensuring compliance with Environmental, Social, and Governance (ESG) regulations.
Timeline: Approved in February 2022. Approved by the European Council March 15, 2024, the next step is parliamentary approval.
Who: Companies with 1000+ employees and a turnover of €450 million.
EU Corporate Sustainability Reporting Directive (CSRD)
Objective: The CSRD aims to harmonize corporate sustainability reporting standards and enhance the availability and quality of disclosed data. Its objective is to provide stakeholders with comprehensive and reliable information on companies’ sustainability performance.
Key Measures: The directive mandates European businesses and subsidiaries of foreign entities to disclose environmental, social, and governance-related information in their annual reports. This includes information on environmental impacts, social indicators, and diversity policies.
Timeline: Implementation begins January 2025, with phased updates.
Who: Large European businesses or European subsidiaries of foreign companies (2025-2026) and SMEs (2027).
EU Deforestation-Free Regulation (EUDR)
Objective: The EUDR seeks to mitigate the risk of deforestation and forest degradation associated with products entering or leaving the EU market. Its objective is to promote sustainable sourcing practices and reduce the environmental impact of commodity production.
Key Measures: The regulation requires businesses to trace the origin of commodities and products, conduct mandatory due diligence, and implement risk mitigation measures. This includes using satellite monitoring tools, conducting field audits, and collaborating with suppliers to ensure compliance with deforestation-free requirements.
Timeline: Enforced since June 29, 2023, with full applicability starting December 30, 2024.
Products: Soy, beef, palm oil, cocoa, coffee, and some of their derived products, such as chocolate. Other non-food products include wood and rubber. See Annex 1 of the regulation.
Who: Companies placing commodities or products in the EU market.
EU Forced Labour Regulation
Objective: The EU Forced Labour Regulation aims to prohibit products made with forced labour from entering the EU market, irrespective of production origin. Its objective is to combat human rights abuses and promote ethical sourcing practices.
Key Measures: The regulation mandates companies to establish robust due diligence practices, implement transparent policies, and ensure auditable traceability across their supply chains. This includes conducting risk assessments, monitoring suppliers’ compliance, and taking corrective actions when violations are identified.
Timeline: Reached agreement on March 13, 2024, with publication imminent and a three-year adaptation period for EU countries.
Products: Products manufactured within the EU for either domestic use or export beyond EU borders, as well as goods imported into the EU, encompassing all industries.
German Supply Chain Act (LkSG)
Objectives: The LkSG seeks to enforce human rights and environmental standards across supply chains by imposing due diligence obligations on enterprises. It advocates for the establishment of robust risk management systems to detect and mitigate potential human rights violations and environmental harm. Furthermore, it emphasizes transparency and accountability through mandatory reporting and complaint mechanisms for enterprises. Additionally, the legislation extends the responsibility of enterprises beyond their direct operations to cover the actions of contractual partners and indirect suppliers throughout the entirety of the supply chain.
Key Measures: Enterprises are required to establish and uphold due diligence obligations aimed at respecting human rights and preventing environmental harm, coupled with mandatory regular reporting to demonstrate compliance. Additionally, complaint procedures must be implemented to address violations. Authorities are empowered with effective enforcement measures to monitor and ensure adherence to supply chain regulations, including the imposition of fines up to 2% of annual global turnover or 8 million euros for larger enterprises. The Federal Office for Economic Affairs and Export Control is granted supervisory powers, enabling them to enter premises, request information, and enforce compliance through financial penalties.
Timeline: Enforced from 1 January 2023, targeting companies with substantial operations in Germany.
Who: German based companies or companies who have a branch in Germany that have: at least 3,000 employees in Germany (2023) and then at least 1,000 members employees in Germany (from 2024)
US Food Safety Modernization Act (FSMA) Food Traceability Final Rule
Objective: The Food Traceability Final Rule aims to strengthen food safety systems by enforcing comprehensive traceability practices across the food supply chain. Its objective is to enhance public health protection and facilitate rapid response to foodborne illness outbreaks and other public health threats.
Key Measures: The implementation of traceability recordkeeping mandates targets individuals involved in manufacturing, processing, packaging, or storing foods listed on the Food Traceability List (FTL), requiring them to maintain records containing Key Data Elements (KDEs) associated with Critical Tracking Events (CTEs). Compliance entails supplying pertinent information to the FDA within 24 hours or within an agreed upon timeframe, encompassing both domestic and international companies contributing to food production for consumption in the United States. The initiative aims to align with industry-leading practices to ensure efficiency across the entire food supply chain, from agricultural production to consumer consumption.
Timeline: Finalised in November 2022, enforceable from January 2026.
Products: Cheese, nut butter, fresh herbs, leafy greens, various types of fresh fruits, finfish, crustaceans, molluscan shellfish, ready-to-eat deli salads and shell eggs.
Who: Extends to all entities, both domestic and foreign, engaged in food production for consumption in the US, excluding small-scale farms.
These regulations, driven by various factors such as the demand for transparency and the fight against environmental crimes, pose significant challenges and opportunities for global supply chains. Transparency remains a critical challenge to be addressed, with profound ethical and business implications. This article underscores the transformative potential of these regulations globally, transcending sectors and geographical boundaries, with repercussions ranging from financial penalties to criminal liabilities.
At Natural Trace, we are proud to contribute to the advancement of traceability and the safeguarding of supply chains. We invite you to reach out to our team to discuss how we can assist you in achieving your transparency objectives and meeting your specific requirements. We are here to ensure every great product comes with a trusted provenance story.
References
https://www.linkedin.com/pulse/recent-regulations-driving-traceability-food-agriculture-gairc
EU Corporate Sustainability Due Diligence Directive (CSDDD)
EU Corporate Sustainability Reporting Directive (CSRD)
EU Deforestation-Free Regulation (EUDR)
https://green-business.ec.europa.eu/deforestation-regulation-implementation_en
EU Forced Labour Regulation
Food Safety Modernization Act (FSMA)
German Supply Chain Act (LKSG)
https://www.csr-in-deutschland.de/EN/Business-Human-Rights/Supply-Chain-Act/supply-chain-act.html








